
Bioquell SeQure
Wall-mounted and compact, Ecolab’s Bioquell SeQure system helps protect your research and production facility from contamination risks.
Benefits of Bioquell SeQure
When you’re running a biological research or production facility, you need a reliable strategy to address contamination risks that could spoil your work. Help control the bioburden risk with the Bioquell SeQure, a compact, fixed system.
Installation and retrofitting of the Bioquell SeQure can be completed and validated in as little as one week.
Use the Bioquell SeQure in:
- Cleanrooms
- Material airlocks and pass-throughs
- Biotech/cell biology labs
- Bacterial/viral vector labs
- Biopharma production facilities
- BSL-3 / BSL-4 labs
Why Choose Bioquell SeQure
Effective
Hydrogen peroxide vapor achieves a 6-log reduction within an enclosed area.
Adaptable
Easily mount on nearly any wall surface. Ideal system retrofitting an existing space or new construction.
Integrated
Connectivity to your building management system or SCADA via Modbus protocol.
Reliable
Validated the desired performance is repeatably achieved.
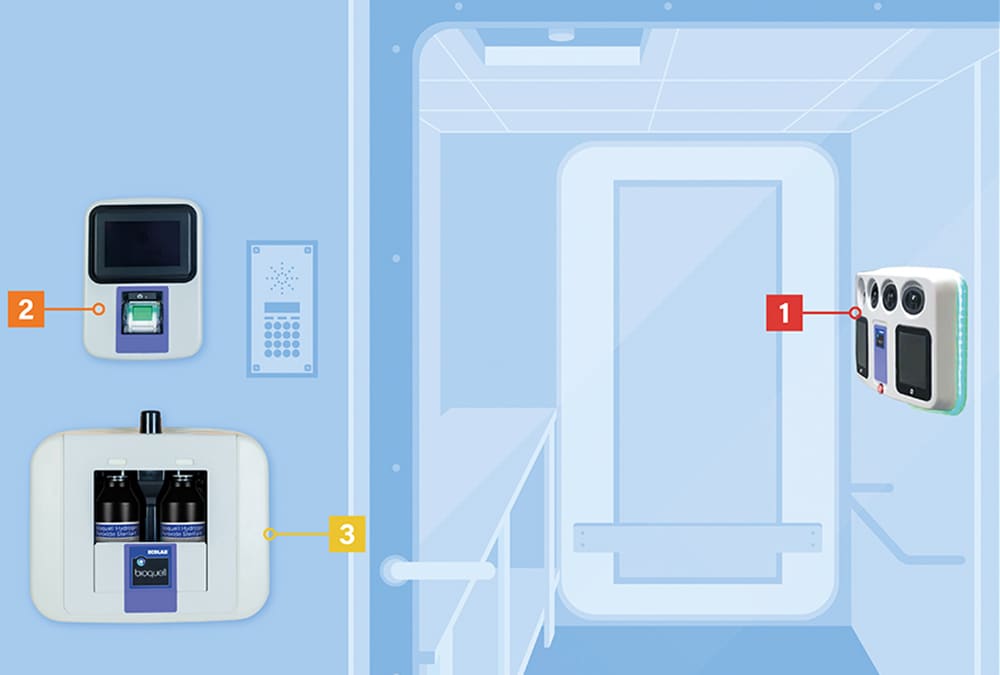
Bioquell SeQure Components
- Hydrogen Peroxide Vaporizer
- 4 integrated fans dynamically distribute vaporized hydrogen peroxide
- Automated cycle prediction technology
- Operates at room temperature conditions, safe for heat-sensitive materials
- Control Unit
- Easy-to-use, color touchscreen
- Password protected programmed cycles
- Dual Bottle Module
- Minimizes peroxide wastage with no decanting
- Houses 2 bottles of Bioquell HPV-AQ (950ml each)
Related Products and Accessories

Aeration Module
This slim wall- or ceiling-mounted unit helps to remove hydrogen peroxide vapor.

Bioquell HPV-AQ
This is a 35% aqueous hydrogen peroxide solution used in Bioquell technology to generate hydrogen peroxide vapor.
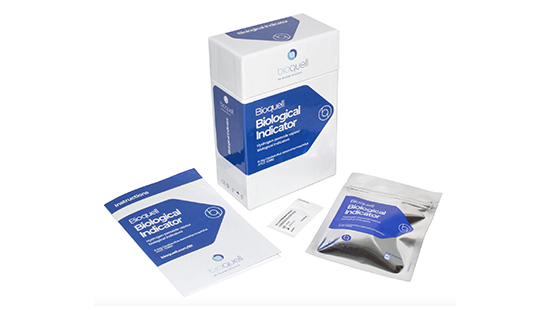
Bioquell Biological Indicators (BIs)
Our biological indicators are made from Geobacillus stearothermophilus endospores to help you validate a Bioquell cycle.
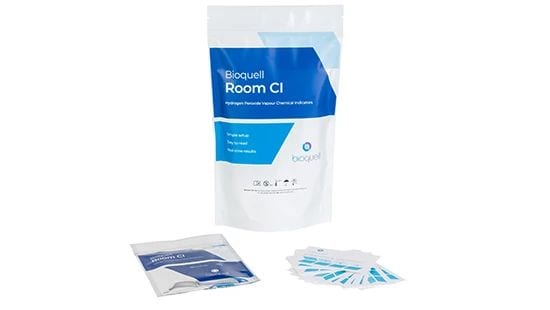
Bioquell Room Chemical Indicators (CIs)
Bioquell Room CIs are our chemical indicators developed for rooms 10m3 and larger. The color-changing strip lets you monitor a cycle in real time.

About Hydrogen Peroxide Vapor
During a bio-decontamination cycle, Bioquell technology fills an enclosed area with 35% hydrogen peroxide vapor. In just one cycle, this vapor provides a 6-log sporicidal kill on exposed surfaces. Unlike chemical sterilants that can leave toxic residues behind, hydrogen peroxide vapor breaks down into water and oxygen once the bio-decontamination cycle and aeration is complete.

Validation
To meet regulatory requirements, you may need to deliver proof that your bio-decontamination solution performs as it should. Ecolab provides trained engineers and the validation documentation you need to comply with regulations.
FAQs About Bioquell SeQure
Can the Bioquell SeQure connect to my Building Management System?
Yes. Our expert technicians will perform a walkthrough of your cleanroom to ensure your existing systems are compatible with the Bioquell SeQure, for integrated HVAC and automated operation.
Bioquell experts also provide continued support for systems that are already in place, ensuring full BMS connectivity and solutions for the lifetime of your system.
I’m wondering if the Bioquell SeQure will fit my space. How big is it?
Vaporizer: 800 x 580 x 160 mm (31.5 x 22.8 x 6.3 in)
Bottle module: 520 x 550 x 140 mm (20.5 x 21.7 x 5.5 in)
Wired control unit: 260 x 350 x 95 mm (10.2 x 13.8 x 3.7 in)
Do you offer 21 CFR Part 11 compliance?
Yes, we can provide an optional data logger for this purpose.


